C O A C H I N G
Insulin
Introduction
The regulation of blood glucose is a very important factor when it comes to health, vitality, and longevity. The pancreas is one of the most , if not the most, important organs when it comes to the regulation of blood glucose. Blood is always flowing through the pancreas, therefore the pancreas always has a gauge upon our blood glucose levels. The pancreas secretes two different hormones, insulin and glucagon, both which help regulate our blood glucose around a homeostatic set point. Excess glucose in the blood stimulates the release of insulin, and inhibits the release of glucagon. Conversely, low levels of blood glucose increase the production of glucagon, and inhibit the production of insulin. Herein we will discuss the regulation of insulin, and the physiological effects of insulin.
Insulin is released by a specific cell within the pancreas referred to as the beta cell. These cells are located in a small subsection of the pancreas referred to as the islets of Langerhans. Insulin is the main hormone responsible for lowering our blood glucose back to normal levels after it has elevated through a myriad of different mechanisms that we will discuss.
Insulin synthesis
Insulin is a protein initially made out of a longer protein referred to as proinsulin. Unbeknownst to many, almost every tissue in the body actually utilizes amino acids. Most people think amino acids are only useful for skeletal muscle, however amino acids are the building blocks for almost every structure, neurotransmitter, hormone, interest cellular protein, receptor, and a whole lot more. In reference to the pancreas, the pancreas can uptake amino acids and use them to produce a large protein referred to as. Proinsulin is stored within a vesicle, where it is subsequently broken down into insulin and C peptide. Upon stimulation, this vesicle releases these 2 proteins into the bloodstream.
I believe the co-release of C-peptide is an important yet underlooked physiological variable. C peptide can be easily measured on a blood test. See that pad remains in the bloodstream much longer than insulin, therefore it may actually be a better indicator of one's total and insulin production, and insulin sensitivity, throughout the day then taking an insulin measurement due to the extremely transient nature of insulin.
Insulin release
Beta cells in the pancreas contain a special type of glucose transporter called a GLUT 2 transporter. At first, you may think that knowing the type of glucose transporter contained on the membrane of the beta cell is not important information. However, the type of glucose transporter is absolutely vital for proper functioning of the insulin system. Glucose transporters on most cells are in the GLUT-4 classification. GLUT-4 proteins are transient membrane-bound glucose channels. What I mean by this is that in order for a glucose transporter to allow glucose to go from the bloodstream into the cell, they must be embedded within the plasma membrane of the cell. GLUT-4 receptors are not permanently embedded in the membrane of the cells. Instead they actually sit inside of the cell, where they don't actually have a function. When stimulated by insulin, muscle contraction, or other hormones, the receptor can translocate (move) from the inside of the cell to the membrane, allowing glucose to enter.
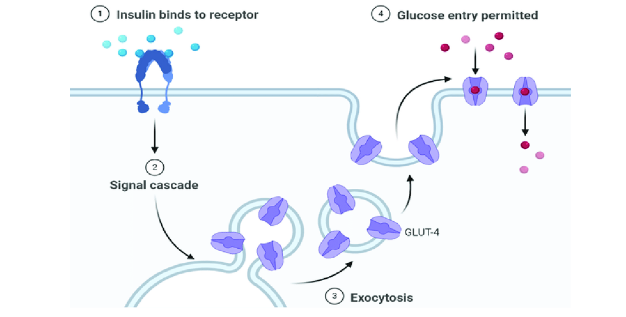
This is a beneficial mechanism for muscle cells, because muscle cells are not glucose dependent. Therefore they do not need to constantly uptake glucose out of the bloodstream to maintain energy homeostasis Conversely, some cells are glucose dependent, most importantly being many cells within the brain and nervous system. GLUT-4 receptors only translocate into the membrane when muscle cells are in need of glucose, and there is enough glucose inside the bloodstream. This allows us to inhibit the uptake of glucose by the muscles in states of low glucose availability in order to spare glucose for the brain.
In order for the pancreas to regulate our blood glucose levels, it needs to constantly be informed of our current levels of blood glucose, thus glucose must always be passing from the bloodstream into the pancreas. Luckily, our physiology is eloquently designed that pancreatic alpha cells express GLUT-2 receptors, which are permanently embedded in the membrane alarm for glucose to enter the pancreatic beta cell in a dose dependent manner depending on our blood glucose levels.
Within the beta cell glucose is used for the production of ATP. The more glucose is in the bloodstream the higher the ATP creation and concentration inside of the beta cells. This accumulation of ATP can bind to potassium channels located on the membrane of the cell, closing the potassium channel which causes potassium to accumulate inside the cell. To frame this discussion I recommend reading my article about glutamate, which goes over the process of cellular excitation and the release of stored molecules. In essence, hormones stored in vesicles are released when the voltage and then the cells surpasses approximately 50 ml. This occurs via the accumulation of positively charged molecules within the cell. When ATP is elevated due to elevations in blood glucose, ATP closes these potassium channels and positively charged potassium accumulates inside the of the cell. This increases the voltage of the cell.
In addition to the potassium channels, there are also what are referred to as voltage gated calcium channels. As the name implies, these cells are highly responsive to an increase in cell charge/voltage, and the potassium buildup causes them to open. The result is the influx of calcium into the cell.
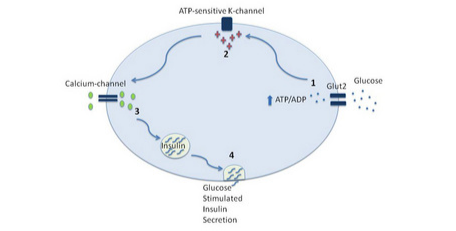
Calcium is the substrate that allows the vesicle containing insulin to fuse the plasma membrane and release the glutamate stored inside of the vesicle. This is mediated by proteins present on the vesicle and on the plasma membrane referred to as SNARE proteins. Calcium allows these proteins to attach to one another, essentially fusing the vesicle into the membrane, and release the stored neurotransmitter, which in this case is glutamate.
Calcium is necessary for allowing the vesicle to fuse to the membrane of the neuron, and release glucagon into the bloodstream. Calcium allows for the interaction of proteins called SNARE proteins, located on the membrane of the vesicle, and the membrane of the cell to bind to one another and fuse the vesicle to the membrane of the cell and release its stored insulin.
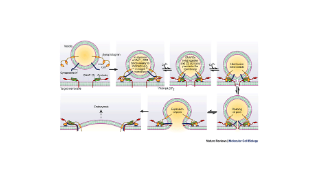
If you're a nerd like me, here is the cellular physiology of this process:
On the membrane of the vesicle, there is a protein called synaptobrevin. Also, on the membrane of the vesicle is another protein called synaptotagmin. On the membrane of the cell, where the vesicle will dock, there’s a protein called syntaxin. Additionally, on the membrane, there is a protein called SNAP-25. Simply, these three proteins form a complex that allows the vesicle to dock on the membrane, until it is ready to be fused into the membrane and undergo exocytosis. Exocytosis is simply the process of releasing the stored insulin from the vesicle into the external environment. Here is where calcium comes in. On the protein we mentioned earlier called synaptotagmin, there are two distinct portions. The C2A portion and the C2B portion. Calcium binds to the C2A portion on the synaptotagmin, then the C2B region will see use into the membrane around the SNARE proteins. While it is slightly unclear the exact mechanism, the synaptotagmin protein causes the membrane of the neuron to begin to curve inwards in the direction of the vesicle. As the membrane of the neuron and the vesicle are both made out of the same phospholipid material, the membranes simply join together, and release the insulin into the external environment.
Simply, elevated ATP stimulated by excess glucose leads to the accumulation of potassium inside the cell, allowing calcium to enter the cell, which is necessary for the vesicle storing insulin to bind to the membrane and release the insulin into the bloodstream.
Insulin receptor
The insulin receptor is profoundly complex and functions through a multitude of different intercellular signaling molecules in order to exert its functions. Through a profound number of different proteins, insulin regulates enzymes, transcription factors, and intracellular proteins. There is such a large abundance of cellular proteins with complex names and roles that listing out all of them often becomes confusing. If you are truly interested in understanding the exact cellular signaling cascade stimulated by insulin, here is a very comprehensive review (1). However, the type of proteins that are either upregulated or downregulated by insulin are different depending on what type of cell insulin is binding to.
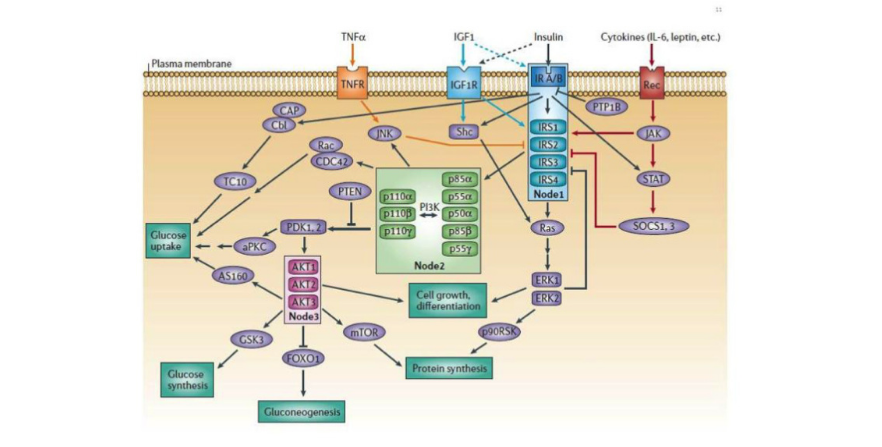
Insulin's effects
Muscle
As mentioned, skeletal muscle contains GLUT-4 glucose transporters. These transporters stay within the cell until stimulated by muscle contraction or chemical messengers. Insulin is the main chemical messenger that causes the translocation of GLUT-4 transporters from the inside of the cell to the membrane of the cell, allowing the muscle to uptake glucose out of the bloodstream. Moreover, insulin stimulates enzymes that function in the utilization of glucose for energy, and upregulate the enzymes responsible for glycogen synthesis. Glycogen is simply the storage form of glucose, which can be broken back down into glucose to be used as energy for muscle contractions.
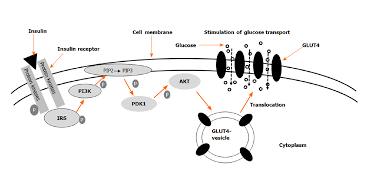
Furthermore, insulin stimulates the uptake and storage of fatty acids in the form of intracellular lipid droplets. These intercellular lipids can actually be used as energy during exercise as just like glycogen.
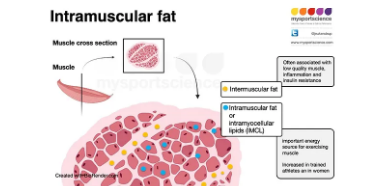
Finally, insulin can stimulate the uptake of amino acids out of the bloodstream, and increase the production of muscle proteins, known as protein synthesis. Protein synthesis is mediated mainly by and intracellular protein referred to as MTOR. This protein not only increases protein synthesis, but it also inhibits protein breakdown. Insulin is oftentimes reffered to as being anti-catabolic, because it will inhibit protein degradation. That said, carbohydrates (therfore insulin) do not have an additve effect on muscle growth when added to a meal that is already sufficient in protein (approximately 20-25 grams of high quality protein) (2).
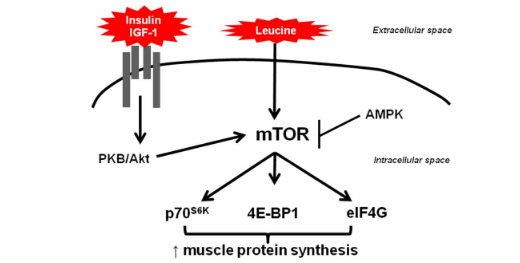
Liver
Within the liver, insulin upregulated enzymes involved in glycogen synthesis, and inhibits the enzymes responsible for glycogen breakdown. Insulin also upregulates enzymes responsible for de novo lipogenesis, or the creation of triglycerides out of alternative fuel sources, namely glucose.
Brain
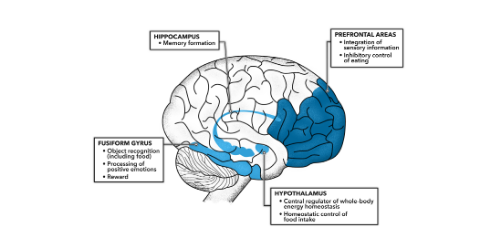
Insulin has a number of fascinating effects in the brain, and researchers are still discovering more and more as time progresses. Insulin stimulates increased fuel uptake and utilization within the neurons, and also stimulates cell growth and proliferation, which is an essential process for learning and memory. Interestingly, insulin can actually be produced inside of the brain, or also transported from the blood into the brain. Insulin production within the brain works through the same mechanism as insulin produced within the pancreas, via glucose stimulation. Brain insulin increases the stimulation of GLUT 4 receptor translocation to the membrane of different neurons such that they can uptake glucose to use for energy in the same way can be uptaken and used within the muscle tissue. With that being said glucose uptake within the neurons is enhanced by insulin, but not dependent on insulin due to the presence of non-insulin dependant glucose channels on most cells within the brain. In fact, most neurons, the cell type in the brain responbsible for sending and receiving chemical messengers called neurotransmitters, do not use glucose. Rather glucose is uptaken into a different cell type referred to as an astrocyte, where it converted into lactate. This asctrocyte derived lactate is subsequently shuttled into neurons to be used as fuel. Consequently, insulin receptors arte mainly located on astrocytes within the brain (3,4).
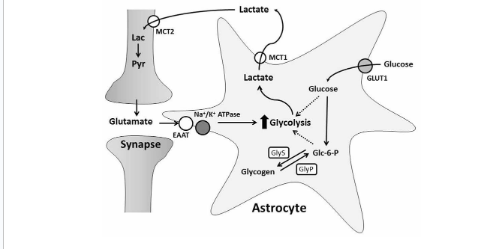
Similar to insulins action on the muscle, insulin stimulates protein synthesis, specifically the synthesis of certain neurotransmitters, and the receptors for these neurotransmitters. For example, insulin can increase the abundance of glutamate receptors within the brain, which is likely the way that insulin can help with memory formation (5).
Moreover, insulin increases leptin production within the fat cell, and leptin works within the brain and nervous system to increase metabolism and decrease appetite. If you would like to learn more about leptin, I have an entire article dedicated to the fascinating function of this signaling molecule. In essence, insulin can increase metabolic rate and decrease hunger by stimulating leptin synthesis.
Insulin can decrease the production of neuropeptide y and AGRP, which are two chemical messengers that will stimulate appetite. Moreover insulin will increase the production of POMC and CART, which is you read my article about leptin you would know these molecules work together it suppress appetite and increrease metabolic rate. Furthermore, insulin can increase the production of GnRH, the initial molecule synthesised in the brain to stimulate the procution of reproductive hormones (6). Insulin has a myriad of other positive and beneficial effects on the brain, which is why complications pertaining to insulin resistance of the brain are such detrimental diagnoses. It is speculated that Alzheimer's may potentially be driven by insulin resistance within the central nervous system and brain.
Kidney
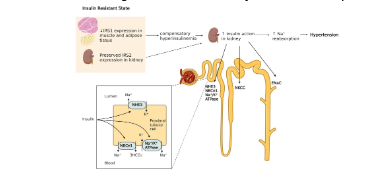
Within the kidney insulin increases sodium absorption by up regulating the production of several different ion channels, particularly for the reabsorption of sodium. This is likely why is very difficult to maintain adequate electrolyte balance on a ketogenic diet, due to insufficient sodium reabsorption within the kidney leading to increased sodium and water loss within the urine. Moreover, one of the hallmarks of the disease characterized by having improper insulin signaling, diabetes, is excessive urination due to insufficient insulin stimulation of the kidneys resulting in excess electrolyte and subsequent water loss do to excess urination (7).
Adipose tissue
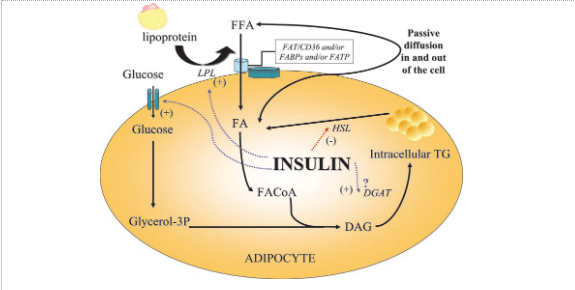
Insulin stimulates the uptake of glucose fatty acids and amino acids at the level of the adipose tissue. They are converted into triglycerides and stored within the fat tissue to be liberated during low energy availability. Instant also inhibits lipolysis, for the breakdown of stored fat.
Insulin resistance
Insulin resistance is characterized by an inability to properly respond to circulating insulin. Individuals with insulin resistance will typically have higher levels of circulating insulin, because theire cells are not adequately responding to the circulating insulin. Insulin resistncae is not an actual diagnosis, rather it lie on a spectrum between the most insulin sensitive to completly resistant to insulin. Diabetes is a diseas characterized by a high degree of insulin reistance which leads to chronically elevated blood glucose and many of the vascular, cardiovascular, and cognitive effects of elevated blood glucose. Moreover, insulin reistnace decrease protein synthesis leading to m,uscle atrophy, decreases glucose availabilty within the cells leading to decreased energy availability within the muscle, organs, and brain. Finally, insulin resistance increases gluconeogensis and glycogen breakdown within the liver due to the inability of the liver to suppress these 2 functions upon insulin stimulation.
Insulin resistance is often misrepresentedas being caused by having too much circulating insulin for a long period of time. However, emerging evidence has been coming out that insulin resistance likely isn't caused by insulin itself, rather it's caused by an accumulation of fat within cells such as the skeletal muscle and liver. When fat accumulates within a tissue, and it is not quickly and efficiently used as energy, fats have the ability to rancidify. This produces fatty acid oxidation products, namely diacylglycerol and ceramides (8). These rancidified fatty acid byproducts oftentimes imp[air proper insulin signaling by binding to the proteins that should respond to insulin. Thus, despite insulin binding to its receptors, be intercellular protein and messaging molecules that should respond by a regulating genetranscription and GLUT 4 receptor translocation become damaged and inhibited by these fatty acid oxidation byproducts. Therefore, despite there being high levels of circulating insulin, the cells are unable to properly respond to insulin binding to its receptor.
This fatty acid accumulation occurs due to a few different lifestyle factors. The first is eating in a caloric surplus therefore storing excessive amounts of fat, namely in the muscles and the liver. The second is lack of physical activity. The reason lack of physical activity causes insulin resistance is because stored fatty acids can be used as energy during physical activity. However, lack of physical activity causes these fatty acids to accumulate inside of the cell. When these fatty acids are left inside of the cell and not utilized for a long period of time, they begin to rancidify and form these fatty acid byproducts that impair insulin signaling.
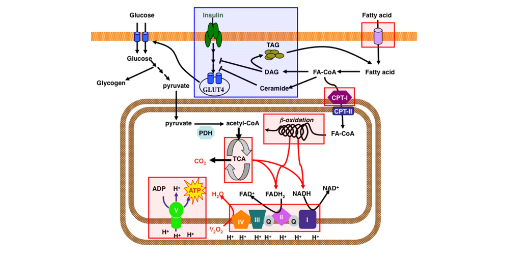
In addition to excessive fatty acid accumulation inflammatory molecules such as IL 6, and other molecules produced in response to inflammation will also decrease insulin sensitivity by disrupting proper insulin signaling. In essence , insulin resistance can be mitigated by reducing inflammation, which can oftentimes be caused by the consumption of foods an individual may be intolerant to, high blood pressure caused by an imbalanced electrolyte intake, underlying infections, pathogens or diseases. Moreover, it can be mitigated by maintaining a healthy body weight and frequent exercise in order to reduce the accumulation of intracellular fat within tissues such as the muscle and the liver.
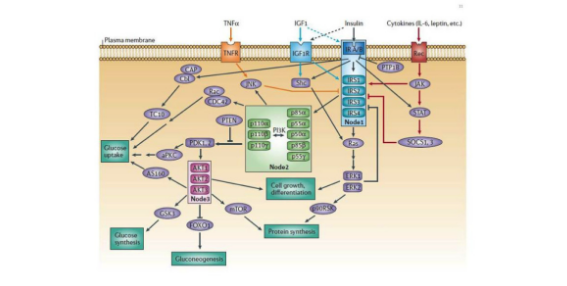
Concluding remarks
Insulin also has some fascinating effects on other organ systems such as the heart, blood vessels, thyroid, adrenal gland, and other tissues within the body. However, these effects listed above are likely the most significant and relevant effects of insulin within the body. With that being said, if you have any questions or think that there are some pertinent effects of insulin that I may not have mentioned, absolutely reach out to me and I will be happy to update this article as often as I need to in order to make it as comprehensive as possible.
https://www.ncbi.nlm.nih.gov/books/NBK378978/ https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3850644/ https://pubmed.ncbi.nlm.nih.gov/34843897/#:~:text=Glucose%20transported%20to%20the%20brain,can%20block%20stroke%2Dinduced%20neurodegeneration. https://www.frontiersin.org/articles/10.3389/fnins.2017.00043/full https://www.ncbi.nlm.nih.gov/pmc/articles/PMC30692/ https://www.embopress.org/doi/full/10.1038/embor.2012.174 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6354081/ https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6712072/#:~:text=Accumulation%20of%20intramuscular%20lipids%2C%20in,the%20induction%20of%20insulin%20resistance.\