C O A C H I N G
Glucagon
Introduction
The regulation of blood glucose is a very important factor when it comes to health, vitality, and longevity. The pancreas is one of the most , if not the most, important organs when it comes to the regulation of blood glucose. Blood is always flowing through the pancreas, therefore the pancreas always has a gauge upon our blood glucose levels. The pancreas secretes two different hormones, insulin and glucagon, both which help regulate our blood glucose around a homeostatic set point. Excess glucose in the blood stimulates the release of insulin, and inhibits the release of glucagon. Conversely, low levels of blood glucose increase the production of glucagon, and inhibit the production of insulin. Herein we will discuss the regulation of glucagon, and the physiological effects of glucagon..
Glucagon is released by a specific cell within the pancreas referred to as the alpha cell. These cells are located in a small subsection of the pancreas referred to as the islets of Langerhans. Glucagon is vital for allowing us to go long periods of time without the consumption of food. For example, overnight we break down about 8% of our liver glycogen every single hour. Glycogen is the storage of glucose found within the liver and muscle tissue. This liver glycogen is released into the bloodstream where this glucose can then be uptaken into cells that use glucose, some of these cells can only use glucose as a fuel source, the most notable being the brain. Glucagon is the predominant hormone that keeps our blood glucose levels stable when we are not eating, or when we are in a carbohydrate depleted state in which we are forced to liberate stored liver glycogen for energy.
Glucagon synthesis
Glucagon is a protein initially made out of a longer protein referred to as pro glucagon. Unbeknownst to many, almost every tissue in the body actually utilizes amino acids. Most people think amino acids are only useful for skeletal muscle, however amino acids are the building blocks for almost every structure, neurotransmitter, hormone, interest cellular protein, receptor, and a whole lot more. In reference to the pancreas, the pancreas can uptake amino acids and use them to produce a large protein referred to as pro glucagon. Proglucagon is converted into glucagon via an enzyme named Prohormone convertase 2 (PC2) enzyme . Glucagon is then stored inside of peptide vesicles within the pancreatic alpha cell. And upon stimulation, the glucagon is released into the bloodstream.
Glucagon release
Alpha cells in the pancreas contain a special type of glucose transporter called a GLUT 1 transporter. At first, you may think that knowing the type of glucose transporter contained on the membrane of the alpha cell is not important information. However, the type of glucose transporter is absolutely vital for proper functioning of the glucagon system. Glucose transporters on most cells are in the GLUT-4 classification. GLUT-4 proteins are transient membrane-bound glucose channels. What I mean by this is that in order for a glucose transporter to allow glucose to go from the bloodstream into the cell, they must be embedded within the plasma membrane of the cell. GLUT-4 receptors are not permanently embedded in the membrane of the cells. Instead they actually sit inside of the cell, where they don't actually have a function. When stimulated by insulin, muscle contraction, or other hormones, the receptor can translocate (move) from the inside of the cell to the membrane, allowing glucose to enter.
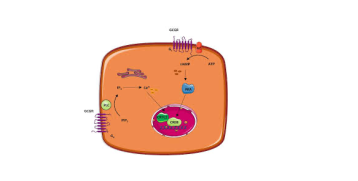
This is a beneficial mechanism for muscle cells, because muscle cells are not glucose dependent. Therefore they do not need to constantly uptake glucose out of the bloodstream to maintain energy homeostasis Conversely, some cells are glucose dependent, most importantly being many cells within the brain and nervous system. GLUT-4 receptors only translocate into the membrane when muscle cells are in need of glucose, and there is enough glucose inside the bloodstream. This allows us to inhibit the uptake of glucose by the muscles in states of low glucose availability in order to spare glucose for the brain.
In order for the pancreas to regulate our blood glucose levels, it needs to constantly be informed of our current levels of blood glucose, thus glucose must always be passing from the bloodstream into the pancreas. Luckily, our physiology is eloquently designed that pancreatic alpha cells express GLUT-1 receptors, which are permanently embedded in the membrane alarm for glucose to enter the pancreatic alpha cell in a dose dependent manner depending on our blood glucose levels.
Within the alpha cell glucose is used for the production of ATP. The more glucose is in the bloodstream the higher the ATP level in the alpha cell. Importantly, ATP inhibits the alpha cell from releasing stored glucagon. This is how high blood glucose can inhibit the release of glucagon, because it increases intracellular ATP levels. Low levels of blood glucose will lower the ATP creation and concentration in the beta cell. To frame this discussion I recommend reading my article about glutamate, which goes over the process of cellular excitation and the release of stored molecules. In essence, hormones stored in vesicles are released when the voltage and then the cells surpasses approximately 50 ml. This occurs via the accumulation of positively charged molecules within the cell. The reason ATP is so important is because ATP will bind toATP sensitive potassium channels, allowing these channels to remain open and release potassium out of the cell. Low levels of ATP, due to low glucose, there is a reduced amount of ATP in the cell, thus causing these potassium channels to close. This resulted in an accumulation of potassium inside of the cell. This is vital because potassium is a positively charged ion. Therefore its accumulation inside of the cell will raise the voltage of the cell.
In addition to the potassium channels, there are also what are referred to as voltage gated calcium channels. As the name implies, these cells are highly responsive to an increase in cell charge/voltage, and the potassium buildup causes them to open. The result is the influx of calcium into the cell.
Calcium is the substrate that allows the vesicle containing glucagonto fuse the plasma membrane and release the glutamate stored inside of the vesicle. This is mediated by proteins present on the vesicle and on the plasma membrane referred to as SNARE proteins. Calcium allows these proteins to attach to one another, essentially fusing the vesicle into the membrane, and release the stored neurotransmitter, which in this case is glutamate.
Calcium is necessary for allowing the vesicle to fuse to the membrane of the neuron, and release glucagon into the bloodstream. Calcium allows for the interaction of proteins called SNARE proteins, located on the membrane of the vesicle, and the membrane of the cell to bind to one another and fuse the vesicle to the membrane of the cell and release it's stored glucagon.
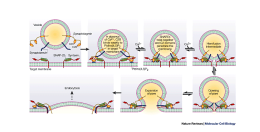
If you're a nerd like me, here is the cellular physiology of this process:
On the membrane of the vesicle, there is a protein called synaptobrevin. Also, on the membrane of the vesicle is another protein called synaptotagmin. On the membrane of the cell, where the vesicle will dock, there’s a protein called syntaxin. Additionally, on the membrane, there is a protein called SNAP-25. Simply, these three proteins form a complex that allows the vesicle to dock on the membrane, until it is ready to be fused into the membrane and undergo exocytosis. Exocytosis is simply the process of releasing the stored glucagon from the vesicle into the external environment. Here is where calcium comes in. On the protein we mentioned earlier called synaptotagmin, there are two distinct portions. The C2A portion and the C2B portion. Calcium binds to the C2A portion on the synaptotagmin, then the C2B region will see use into the membrane around the SNARE proteins. While it is slightly unclear the exact mechanism, the synaptotagmin protein causes the membrane of the neuron to begin to curve inwards in the direction of the vesicle. As the membrane of the neuron and the vesicle are both made out of the same phospholipid material, the membranes simply join together, and release the glucagon into the external environment.
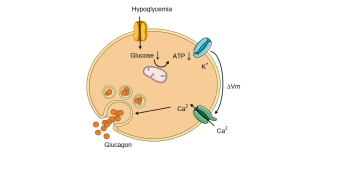
Simply, low ATP leads to a potassium buildup inside the cell, allowing calcium to enter the cell, which is necessary for the vesicle storing glucagon to bind to the membrane and release the glucagon into the bloodstream.
Glucan receptor
Glucagon exudes effects on all cells through the G-protein coupled receptors. Glucagon binds to a g-protein receptor on the outside of the membrane. Attached on the inside of the membrane is a G-stimulatory protein. In its “off state” G-stimulatory proteins are bound to GDP. When glucagon binds to its receptor, the GDP is released, and a different molecule called GTP will bind.
The GTP complex activates an enzyme called adenylyl cyclase, which will convert ATP into cAMP. cAMP will cause a protein in the cell called PKA to go into the nucleus of the cell where our DNA is stored, and activate transcription factors. These are small proteins within the nucleus that will find the DNA and increase the production of specific proteins that are important for cellular function. Glucagon has the same effect of altering protein production through interacting with transcription factors within every cell. However, the type of proteins that are either upregulated or downregulated by glucagon aredifferent depending on what type of cell glucagon binds to.
Glucagon actions
Muscle
Glucagon will increase the breakdown of muscle tissue, referred to as muscle tissue catabolism, in order to release the amino acids found in muscle tissue into the bloodstream, which they can subsequently travel to the liver and be turned into glucose through a process called gluconeogenesis. Increasing amino acid availability in the bloodstream will increase gluconeogenesis of amino acids, therefore aiding in returning blood glucose glucose back to homeostatic levels, and maintaining it there (1).
Adipose tissue
Glucagon receptors on the fat tissue enhance the enzymes responsible for breaking down store fat. Restore fat in the form of a triglyceride within adipose tissue. Glucagon up regulates the enzymes that are capable of breaking down triglycerides into its usable components, which are glycerol and three fatty acids. The glycerol and three fatty acids are released into the bloodstream. The free fatty acids can be directly used as energy by tissues that can oxidize fatty acids, and the glycerol will travel through the bloodstream to the liver where it can be turned into glucose and released back into the bloodstream. With this mechanism, we can increase blood glucose via glycerol conversion into glucose, and preserve blood glucose by increasing the availability of fatty acids in the bloodstream which can be used in place of glucose for energy (1).
Liver
Glucagon regulates a vast array of different enzymes in the liver. Glucagon can stimulate the upregulation of enzymes responsible for the breakdown of glycogen, and inhibit the enzymes responsible for creating glucose. Thus, having the overall effect of inhibiting the storage of glucose, and increasing the release of glucose into the bloodstream, both of which increase and maintain blood glucose levels.
Glucagon upregulates enzymes responsible for lipolysis, or the breakdown of triglycerides in the liver into their usable components of glycerol and free fatty acids. Just as important, glucagon downregulated lipogenic enzymes, which are responsible for the conversion of glucose into fatty acids. It also decreases the production of VLDL, the lipoproteins responsible for exporting fat out of the liver, mainly for storage within adipose tissue and muscle.
Glucagon upregulates the production of enzymes responsible for gluconeogenesis, which is the creation of glucose out of non-glucose molecules, namely amino acids, glycerol, and lactate.
Finally, glucagon increases the enzyme responsible for ketogenesis, the production of ketones. As discussed, glucagon increases the release of fatty acids from the adipose tissue. Importantly, similar enzymes are required for both gluconeogenesis, and for the oxidation (burning) of fatty acids inside the liver. This results in an accumulation of a partially broken down fatty acid called acetyl-coa inside the liver. Luckily, acetyl-coa molecules can be transformed into ketones within the liver, which can be released into the bloodstream to be used as an alternative energy source for almost every cell in the body. Glucagon increases the abundance of acetyl-coa in the liver to upregulates release of fatty acids and other acetyl-coa precursors into the bloodstream, and it also increase ketogenic enzymes so we can support both gluconeogenesis and ketogenesis within the liver.
All of these enzymes and proteins work in tandem to increase the release of glucose into the bloodstream, and increase the release of alternative fuels sources as well, being fatty acids and ketones (1).
Heart
Glucagon given via injection increases contractility of the heart, meaning it can increase heart rate. The etiology of this effect isn't entirely understood, however it may be a beneficial adaptation to get more blood around the body to increase the speed of substrate release and its conversion into usable glucose, fatty acids, and ketones (2).
Kidney
Glucagon appears to have the ability to increase kidney function. That is, it increases kidney filtration and excretion of metabolites into the urine. This is beneficial because with an excess of gluconeogenesis from amino acids, there's an excess of a byproduct produced known as ammonia. In excess concentrations ammonia can become damaging, therefore increasing ammonia release in the urine allows us to maintain blood ammonia levels within a safe range (2).
Concluding remarks
Glucagon certainly is permissive of weight loss, particularly in the form of body fat. However, as one can see, excess glucagon can also stimulate the breakdown of muscle tissue. Interestingly, insulin, the hormone that opposes glucagon, can actually inhibit the production of glucagon. With that being said, an increasing percentage of the population is becoming insulin resistant, therefore they lack the ability to inhibit glucagon even when blood glucose is elevated at its worst, this is referred to as type 2 diabetes. The result is an inability to inhibit glucagon production despite elevated blood glucose. This results in chronically elevated blood glucose levels, and excessive amounts of glucose production and release by the liver despite already elevated blood glucose levels. Additionally, this increases the catabolism of muscle tissue to aid the glucagon stimulated gluconeogenesis. Thus, diabetics and individuals with poor insulin sensitivity suffer from muscle wasting and chronically elevated blood glucose, which leads to many of the complications seen in diabetics.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6312800/#:~:text=Glucagon%20opposes%20hepatic%20insulin%20action,amino%20acids%20as%20gluconeogenic%20precursors. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5002329/#:~:text=Glucagon%20and%20the%20heart&text=In%20the%20non%2Dfailing%20heart,and%20oxygen%20consumption%20%5B12%5D.